Sustained acoustic medicine allows people to effectively and easily manage arthritis and soft-tissue pain.
TRUMBULL, CONNECTICUT / ACCESS Newswire / August 11, 2025 / Osteoarthritis, the most common form of arthritis, affects more than 63 million adults nationwide. The sam® wearable ultrasound unit, which recently received Ease of Use Certification from the Arthritis Foundation, allows arthritis patients to manage the painful inflammatory disease from home.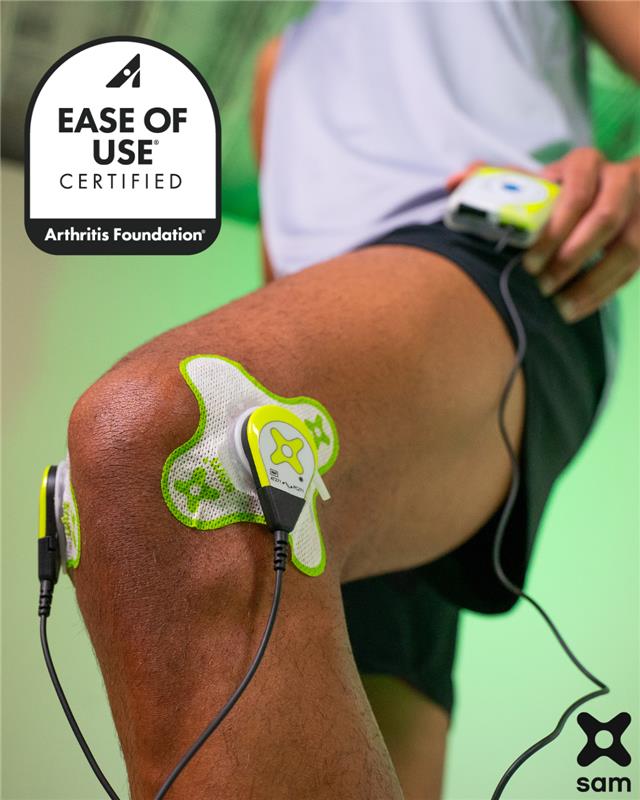
The sam® device, developed and manufactured by Connecticut-based ZetrOZ Systems, is cleared by the U.S. Food and Drug Administration for the treatment of osteoarthritis and other soft-tissue conditions. The device delivers sustained acoustic medicine (sam®), a form of continuous long duration ultrasound, directly to the site of pain or injury. The non-invasive treatment accelerates healing and reduces pain associated with arthritis, muscle strains, and joint injuries.
The sam® device recently received the Arthritis Foundation's Ease of Use® Certification, which signifies that rigorous independent testing confirms that the device can be operated easily by people with limited dexterity or mobility. That means arthritis patients can receive treatment at home, reducing the need for potentially challenging trips to medical care facilities.
"Arthritis care doesn't need to be confined to clinic appointments," said Dr. George K. Lewis, president and CEO of ZetrOZ Systems and the inventor of sustained acoustic medicine. "With sam®, patients have access to real, evidence-backed treatment on their schedule, wherever they are."
Sustained acoustic medicine and the sam® device work by generating continuous ultrasound waves in soft tissue, which reduces inflammation, increases blood vessel diameters, and improves blood flow. That in turn increases oxygenated hemoglobin at the site and removes cytokine enzymes and cellular waste. The result is more rapid healing and reduced pain.
Backed by clinical research and trusted by healthcare providers, the sam® device is redefining the future of conservative arthritis care. A Level 1A clinical study, published in 2020 in the Journal of Orthopaedic Surgery and Research, studied the medical and economic effectiveness of using sustained acoustic medicine to treat knee osteoarthritis (OA).
The researchers, affiliated with the University of Miami, the Albert Einstein College of Medicine and the Orthopaedic Foundation, found that "SAM treatment was more effective in pain and function outcomes for knee OA" compared to standard treatments, and "could provide significant cost savings for a life-long progressing disease."
The researchers also noted the importance of sustained acoustic medicine offering a way to treat arthritis pain without the use of potentially addictive painkillers. "For this very reason," the authors wrote, "SAM treatment should be considered a viable, prescribed, and covered home therapy."
The effectiveness of sustained acoustic medicine and the sam® device is validated by more than 30 clinical studies and more than 3.7 million treatments of patients to date. Whether managing early morning stiffness, post-exercise soreness, or chronic flare-ups, sam® gives arthritis patients the power to treat pain proactively, consistently, and comfortably.
"Arthritis pain doesn't wait, and neither should relief," Lewis said. "We're proud to offer a science-driven solution that empowers patients to take control of their recovery -- safely and effectively from home."
For more information, visit www.zetroz.com.
About ZetrOZ Systems
ZetrOZ Systems is leading healing innovations in sports medicine, developing wearable bioelectronic devices to deliver sustained acoustic medicine (sam®). Researched and funded by the federal government, ZetrOZ is built on the proprietary medical technology of 48 patents and is the exclusive manufacturer and developer of the sam® product line, designed to treat acute and chronic musculoskeletal conditions.
Contact Information
Catherine Hoblin
Media Contact
choblin@zetroz.com
SOURCE: ZetrOZ Systems
View the original press release on ACCESS Newswire