HERZLIYA, ISRAEL / ACCESSWIRE / October 19, 2023 / Induced pluripotent stem cells (iPSCs) have emerged as a promising tool in the field of research and therapy for neurodegenerative diseases such as Amyotrophic Lateral Sclerosis (ALS). ALS is a progressive disorder that affects motor neurons, leading to muscle weakness, paralysis and ultimately death. iPSCs are adult cells that have been reprogrammed to behave like embryonic stem cells, capable of differentiating into various cell types. By generating iPSCs from people living with ALS, researchers are able to study disease-specific cellular pathologies and gain insights into the underlying mechanisms of the disease.
One of the major advantages of using iPSCs in ALS research is the potential to model disease progression and test potential therapeutics in a laboratory setting. iPSCs can be differentiated into motor neurons - the key cells affected in ALS - allowing researchers to study the dysfunction and degeneration of these cells. By comparing iPSC-derived motor neurons from ALS patients to healthy controls, scientists can identify disease-associated changes, such as altered gene expression or protein levels. This can help in the development of biomarkers for early detection and monitoring of ALS progression, as well as provide targets for potential drug development.
One company that is exploring the relationship between iPSCs and ALS is NeuroSense Therapeutics (NASDAQ: NRSN). NeuroSense Therapeutics is a cutting-edge biotechnology company that develops a novel combination therapy aimed to address several mechanisms in this complex disease. NeuroSense, in a collaboration with the University of Southern California (USC), utilized iPSCs to advance the understanding of ALS by studying disease-specific cellular models derived from iPSCs.
PrimeC is specifically designed to treat ALS by modulating microRNA synthesis, reducing neuroinflammation and impacting iron accumulation. This innovative formulation combines meticulously calibrated doses of two FDA-approved drugs, Ciprofloxacin and Celecoxib, with the goal of synergistically inhibiting the advancement of ALS. PrimeC effectively attenuates motor neuron degeneration and suppresses inflammatory responses, surpassing the performance of traditional ALS treatments in studies conducted using a zebrafish model.
The company made an important announcement early this month about its latest development in treating neurodegenerative diseases. An independent study led by Dr. Justin Ichida at USC focused on ALS. Dr. Ichida is a respected expert in stem cell biology and regenerative medicine, known for his innovative research for finding treatments for ALS and Alzheimer's disease. He's regarded as a leader in using iPSCs, a cutting-edge cellular technique, for drug testing and development.
"Dr. Ichida has been described as being beyond the cutting edge in his field and we are honored that he and his lab chose to evaluate PrimeC in this non-sponsored study," shared NeuroSense Founder and CEO Alon Ben Noon. "These results further bolster our confidence that PrimeC may offer a much-needed therapy for this debilitating disease which is ALS. We are proud that PrimeC is recognized as a leading ALS drug candidate by the top medical institutions in the world."
In the study, Dr. Ichida used iPSCs derived from blood samples of ALS patients. The goal was to test the effect of NeuroSense's treatment, PrimeC, on induced motor neurons. The results demonstrated that PrimeC had a significant positive impact on the survival rate of the motor neurons compared to each component alone. This shows the value of combining the two components in NeuroSense's formulation. In addition, according to a previous independent study conducted by Dr. Ichida, PrimeC was among the best molecules that were tested, including two FDA-approved drugs for ALS, as the results were comparable to a healthy control. (see chart below).
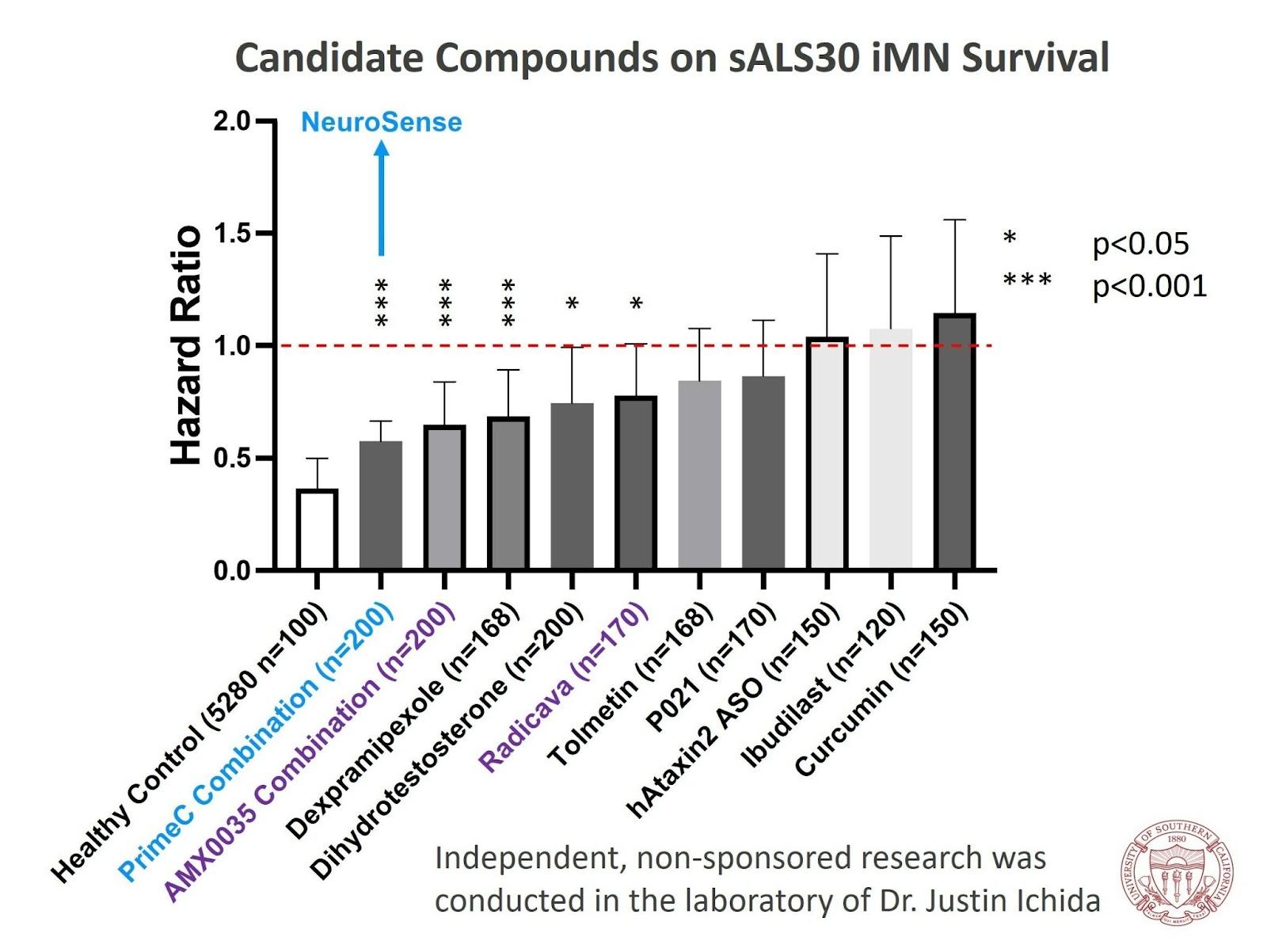
"At our lab, we screen thousands of compounds in search of one that may be effective in ALS, and we were very impressed by the data resulting from our iPCS in vitro study of PrimeC. We chose to evaluate PrimeC based on the growing body of clinical, pre-clinical, and biomarker data on its efficacy in ALS," said Dr. Ichida. "As a candidate compound, NeuroSense's combination was among the best in improving motor neuron survival. Furthermore, in a follow-on study, where we explored specifically the synergistic effect of PrimeC combination relative to each one of its therapeutic agents, the results exceeded our expectations, as PrimeC increased the survival rate to the level of the healthy control and that got us very excited."
This latest study is a significant development for NeuroSense. It provides further evidence of the potential effectiveness of its treatment, PrimeC, in addressing ALS, which is a major breakthrough for the company. NeuroSense is expecting to announce clinical topline results from its phase 2b ALS study in several weeks.
Featured photo by Rattiya Thongdumhyu on Shutterstock.
Contact:
IR: Corporate Profile - Dilek Mir
dmir@corporateprofile.com
SOURCE: NeuroSense Therapeutics Ltd
View source version on accesswire.com:
https://www.accesswire.com/794636/renowned-als-researcher-confirms-the-efficacy-of-neurosense-therapeutics-lead-drug-candidate-primec




