The final results of the physician-led National Cardiogenic Shock Initiative (NCSI) Study demonstrate a 71% survival to discharge with greater than 90% native heart recovery when best practices are used, including placement of an Impella heart pump prior to revascularization (PCI). The study evaluated the outcomes of 406 consecutive patients who presented with acute myocardial infarction cardiogenic shock (AMICS) at 80 participating sites throughout the United States. The study shows a protocol-based approach to increasing survival rates in cardiogenic shock is reproducible in both academic and community hospital settings. Results were presented today by Babar Basir, DO, director of the acute mechanical circulatory support program at Henry Ford Hospital and co-principal investigator of the study, at the 2021 Society of Cardiovascular Angiography & Interventions (SCAI) Scientific Sessions.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210428006079/en/
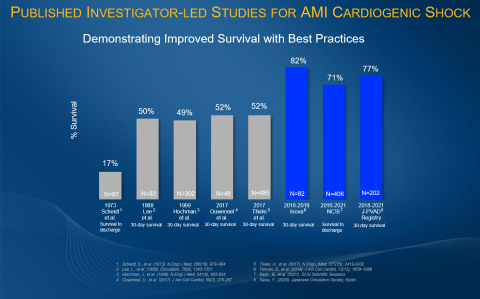
The NCSI Study is a single-arm, prospective study assessing outcomes associated with early mechanical circulatory support (MCS) in AMICS patients treated with PCI. The goal of the NCSI Study is to increase cardiogenic shock survival, which has historically been approximately 50%, by providing early unloading therapy and allowing the heart to rest prior to PCI, promoting native heart recovery. The NCSI Study prospectively validated the previously identified best practices of identifying shock early, placing Impella support pre-PCI within 90 minutes of arriving at the hospital, using a pulmonary artery catheter to guide decision-making, and reducing the use of inotropes and vasopressors.
The study investigators categorized patients according to SCAI Shock classification stages. For SCAI stages C/D, they found higher survival to discharge (79%), 30-days (77%) and 1-year (62%) compared to historical data in similar patients during the last 30 years. SCAI stage E patients, who are on the brink of cardiovascular collapse, also had improved survival to discharge (54%), 30-days (49%), and 1-year (31%) compared to recent studies of patients who were not treated with the NCSI protocol that published in Journal of American College of Cardiology (33% survival to discharge) and Catherization and Cardiovascular Interventions (22.6% survival to 30-days).
“The NCSI Study demonstrates the benefit of following a protocolized approach to AMICS, which includes early implantation of Impella. We are impressed with the improved survival rates seen with the use of best practices compared to the stagnant historical survival rate,” said Dr. Basir. “It is important to emphasize that the majority of hospitals in the NCSI Study were large community-based programs where heart attack patients first present. Giving these care teams a safe and effective protocol to manage critically ill patients has saved many lives.”
“NCSI is the largest prospective study of therapy for AMICS done in the U.S. in the last 20 years. We have found that the original observations from the Detroit Cardiogenic Shock Initiative have been reproduced showing significantly higher survival rates,” said William O’Neill, MD, medical director of the Henry Ford Health System Center for Structural Heart Disease and co-principal investigator of the study. “If implemented across the country, the NCSI protocol can save 20,000 lives a year.”
Other investigator-led research demonstrates results similar to the NCSI Study results, including the 2019 Inova Study and the 2020 J-PVAD Study. These studies found an 82% and 77% survival at 30 days, respectively, when best practices were followed including the early identification of cardiogenic shock and the early use of Impella. (see figure 1)
“The NCSI Study has shown that, through rapid identification and treatment of patients who will benefit from mechanical circulatory support, higher cardiogenic shock survival rates can be achieved,” said Andrew M. Goldsweig, MD, MS, an assistant professor of interventional cardiology and associate catheterization lab director for structural heart disease at the University of Nebraska Medical Center.
The authors concluded early use of mechanical circulatory support and hemodynamics is associated with improved outcomes. Additional clinical research in the treatment of cardiogenic shock includes the RECOVER IV RCT, which is being developed and will be a prospective, two-arm trial to assess whether Impella pre-PCI is superior to PCI without Impella in AMICS patients. In addition, the DanGer Shock RCT is an ongoing trial in Denmark and Germany assessing primary outcome of death from all causes at six months with Impella CP, compared to standard of care.
To watch Dr. Basir and Dr. O’Neill discuss the NSCI Study results, and the ability of the NCSI protocol to improve outcomes for patients, please visit HeartRecovery.com.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI), such as stenting or balloon angioplasty, to reopen blocked coronary arteries.
The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5® with SmartAssist® are U.S. FDA approved to treat heart attack or cardiomyopathy patients in cardiogenic shock and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.abiomed.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support and oxygenation. Our products are designed to enable the heart to rest by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit: www.abiomed.com.
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements. Forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210428006079/en/
Contacts
Media:
Sarah Lima
Communications Manager
978-882-8211
slima@abiomed.com
Investors:
Todd Trapp
Vice President and Chief Financial Officer
(978) 646-1680
ttrapp@abiomed.com





