An analysis of more than 2,000 patients from a large U.S. real-world database finds that patients who underwent a non-emergent high-risk PCI with the Impella heart pump (n=1,447) had significantly improved survival, reduced myocardial infarction, reduced cardiogenic shock after PCI and shorter length of stay than matched patients treated with an intra-aortic balloon pump (IABP) (n=709). The study’s findings are consistent with and reinforce the results of other peer-reviewed studies over the past 10 years.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221013005349/en/
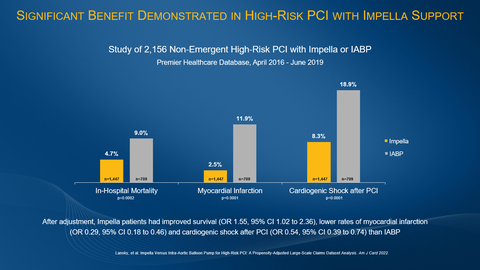
Figure 1 (Graphic: Business Wire)
The study published October 7 in The American Journal of Cardiology. It was conducted by a group of expert physician-researchers, led by Alexandra Lansky, MD, professor of medicine at the Yale School of Medicine and a practicing cardiologist at Yale-New Haven Hospital. Dr. Lansky is also the director of the Yale Heart and Vascular Clinical Research Program and the Yale Cardiovascular Research Center, which specializes in the evaluation of interventional devices.
“This study from a large contemporary, real-world database is further evidence of the benefits of using Impella during high-risk PCI to stabilize hemodynamics, prevent hemodynamic collapse, enable optimal revascularization and improve clinical outcomes,” said Dr. Lansky.
The study examined patients in the Premier Healthcare Database treated between 2016 and 2019 with Impella or IABP for non-emergent high-risk PCI. Patients were matched using propensity-score methods to control for baseline, procedure and post-PCI medical treatment differences between the groups. The study found:
- In-hospital survival was significantly higher with Impella compared to IABP (unadjusted 95.3% vs. 91.0%, p=0.0002; adjusted odds ratio (OR) 1.55, 95% confidence interval (CI) 1.02, 2.36, p=0.042).
- Myocardial infarction was significantly reduced with Impella compared to IABP (unadjusted 2.5% vs. 11.9%, p<0.0001; adjusted OR 0.29, 95% CI 0.18, 0.46, p<0.0001).
- Cardiogenic shock after PCI was significantly reduced with Impella compared to IABP (8.3% vs. 18.9% p<0.0001; adjusted OR 0.54, 95% CI 0.39, 0.74, p=0.0001).
- A shorter length of stay for Impella patients compared to IABP patients (unadjusted 3.1 days vs. 5.5 days, p<0.0001; adjusted 3.4 days vs. 4.8 days, p<0.0001).
Furthermore, the safety profile for bleeding and stroke were the same between the Impella and IABP groups. This is consistent with data demonstrating improvements in bleeding over time for large-bore devices, with the adoption of contemporary practices such as ultrasound-guided vascular access and optimal use of closure devices. (see figure 2)
The authors of Lansky et al. analyzed contemporary payer data from the years after Impella’s FDA approval for high-risk PCI, an era when best practices for Impella use had been established. Additionally, they used a more straightforward propensity-matched methodology than prior studies from similar datasets.
The results of Lansky, et al. further validate and extend the results of other studies of Impella in high-risk PCI, including the PROTECT II randomized controlled trial (RCT), the PROTECT III study and the RESTORE EF study. The PROTECT II RCT found Impella use led to a 29% relative risk reduction in MACCE at 90 days when compared to IABP. The PROTECT III study demonstrated a further improvement in 90-day clinical outcomes, completeness of revascularization, and safety when compared to the PROTECT II RCT. (see figure 3) Restore EF demonstrated the use of contemporary best practices with Impella in high-risk PCI significantly improves left ventricular ejection fraction (LVEF), heart failure symptoms, and anginal symptoms at 90-day follow-up in patients treated at a variety of hospital settings including rural, urban, community and academic medical centers. (see figures 4 & 5)
ABOUT IMPELLA HEART PUMPS
Impella 2.5® and Impella CP® are U.S. FDA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI), such as stenting or balloon angioplasty, to reopen blocked coronary arteries.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed (Nasdaq: ABMD) is a leading provider of medical technology that provides circulatory support and oxygenation. Our products are designed to enable the heart to rest and recover by improving blood flow and/or provide sufficient oxygenation to those in respiratory failure. For additional information, please visit abiomed.com.
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in Abiomed's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221013005349/en/
Contacts
Media Contact:
Jenny Leary
Associate Director, U.S. Communications
+1 (978) 882-8491
jleary@abiomed.com
Investor Contact:
Todd Trapp
Executive Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com





