(BPT) - Antibody testing has become a focal point for policymakers and consumers alike in the ever-evolving conversation around managing the SARS-CoV2 pandemic. As of June 4, the United States Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA), which means the test was reviewed and authorized by the agency, to less than 20 antibody tests.
Because of the immediate need for tests, the FDA established guidelines that allowed companies to market their tests shortly after they developed them. While the FDA has since strengthened its guidance and required all test manufacturers to seek EUA, there are still many antibody tests on the market that have not earned the distinction. This means patients, caregivers, clinicians and government officials could be making important decisions based on tests lacking both quality and accuracy.
“As the world begins to reopen, antibody tests are a critical tool to understand who may have been exposed to the virus and may have developed an immune response,” said Chockalingam Palaniappan, Chief Innovation Officer at Ortho Clinical Diagnostics. “When people are tested with a lab-quality test that has achieved EUA, like those manufactured by Ortho Clinical Diagnostics, they can have confidence that the results they receive are accurate.”
What are antibody tests?
Diagnostic tests, like those performed at a doctor’s office or at a drive-through testing site with a nasal or throat swab, identify the active virus present in the body. Antibody tests, using a finger prick or vial of blood, identify antibodies generated by the body to fight off the disease caused by SARS-CoV2. Early research is being advanced to confirm whether the presence of antibodies in the blood means a patient cannot be re-infected or may have a better chance of beating the virus in the future. Research is also underway to determine how long the benefits of antibodies last.
How do lab-quality antibody tests work?
Once a person’s blood sample is collected and sent to a lab, the sample tube is centrifuged (spun out) to separate the blood into serum (liquid) and blood (plasma). Lab technicians load the tubes into a large instrument, called an analyzer, that runs multiple tests at one time. Lab-produced tests, such as Ortho Clinical Diagnostics’, contain small proteins from the COVID-19 virus. When the viral proteins mix with a sample of the person’s serum, the antibodies in the sample bind to the proteins and cause a chemical reaction, which is measured by the analyzer. The analyzer reports results as reactive (meaning the patient has COVID-19 antibodies) or non-reactive depending on the presence or absence of antibodies in the sample. The analyzer then sends the data directly to the hospital or lab’s information technology team, which provides the results.
What does antibody testing mean for the general public?
SARS-CoV-2 is a new virus and significant research is needed to better understand it. Lab technicians and virologists are hard at work studying the immune system’s antibody response, including how long the presence of antibodies in the blood may help “protect” someone from the virus. In illnesses such as chickenpox or the measles, the presence of antibodies can suggest that immunity will last a lifetime, while in others, like the rhinovirus, immunity can lapse after several weeks.
“Anyone who thinks they may have been exposed to COVID-19 should speak with their clinician to determine if they should get an antibody test,” said Chockalingam. “In this conversation, you should discuss the need for a serological test for COVID-19 with your physician and make sure a lab-quality test with EUA is selected so both you and your clinician can feel confident in the results.”
Speak with your healthcare provider for more information, or visit the CDC website to learn how antibody testing can impact you and your family.
For more information on Ortho’s COVID-19 test, visit www.orthoclinicaldiagnostics.com.
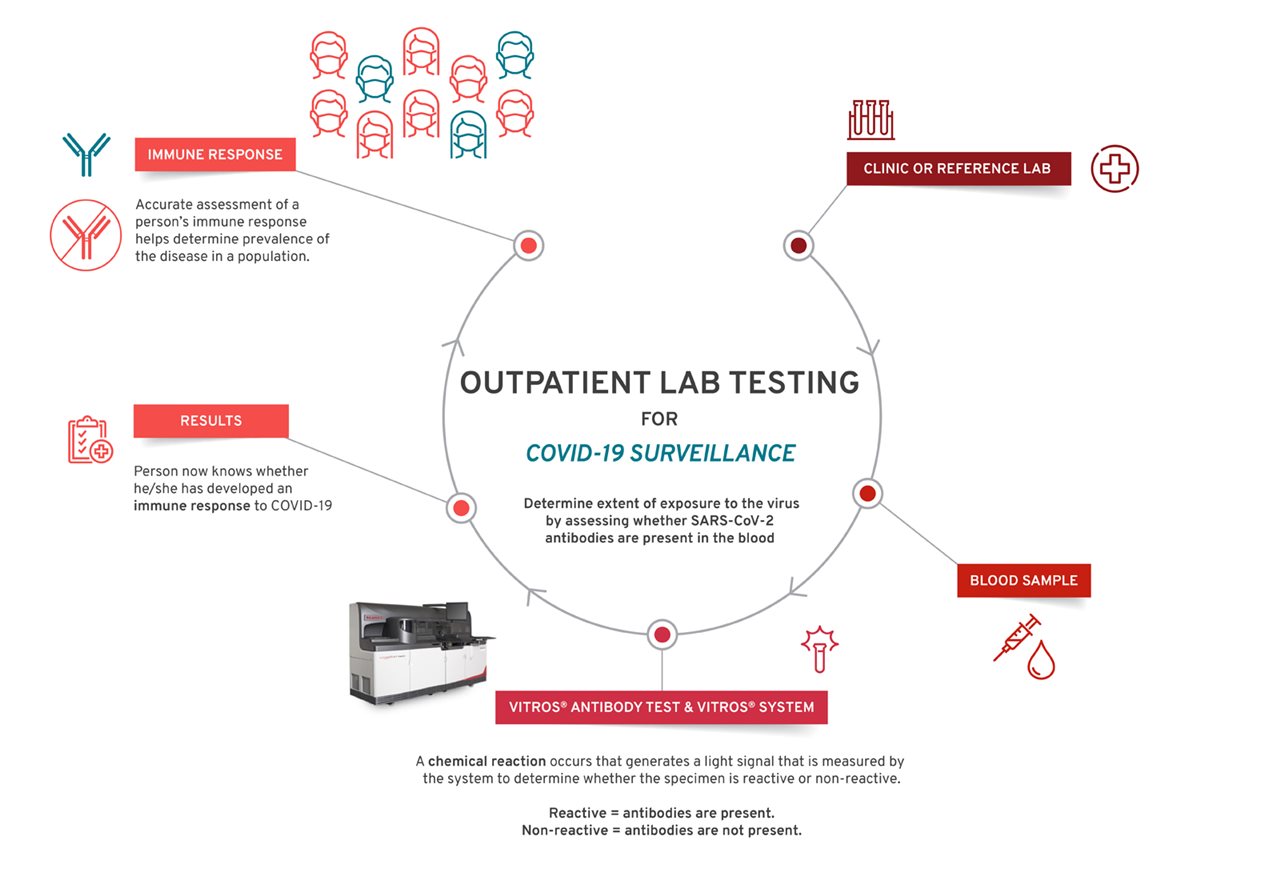
Several steps follow a patient’s journey from hospital admission to determining an immune response (shown above). The Centers for Disease Control and Prevention (CDC) also provides helpful guidance on interpreting COVID-19 test results and who should be tested.

